Which of the Following Has the Lowest Freezing Point
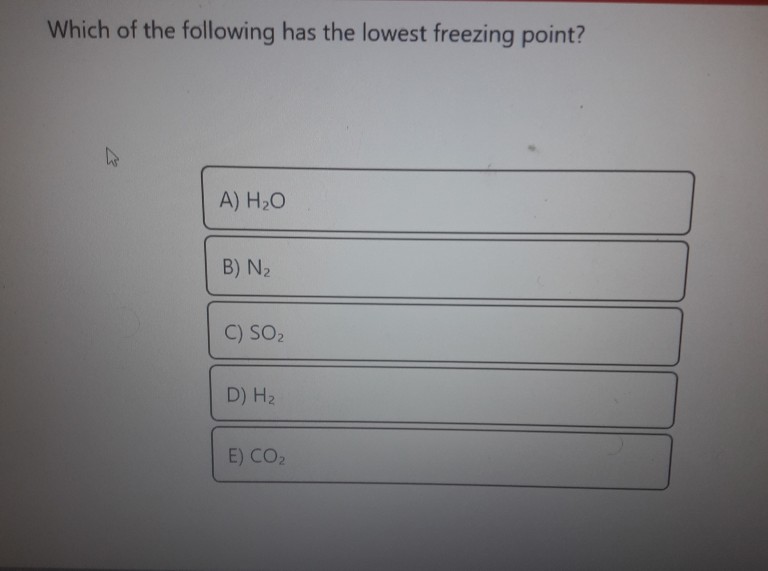
AHO B N 2 C SO DH E COZ. Freon 22 has the lowest freezing point temperature from all of.

When Cuso4 Is Added To A Solution Of Ammonia
A 02 m NaCl B 02 m CaCl2 C 02 m H2SO4 D 02 m NH3 E 02 m AlNO33 Answer and Explanation.
. I know the answer is d because our professor told us so but he did not explain why or how I would calculate to find the answer could you please explain. The solution of BaOH2 will have the lowest freezing point. The molal boiling point constant of water is 051 oCm The molal freezing point constant of water is.
Which one of the following 006 M aqueous solutions has lowest freezing point. Lower freezing point and a lower boiling point. Chemistry questions and answers.
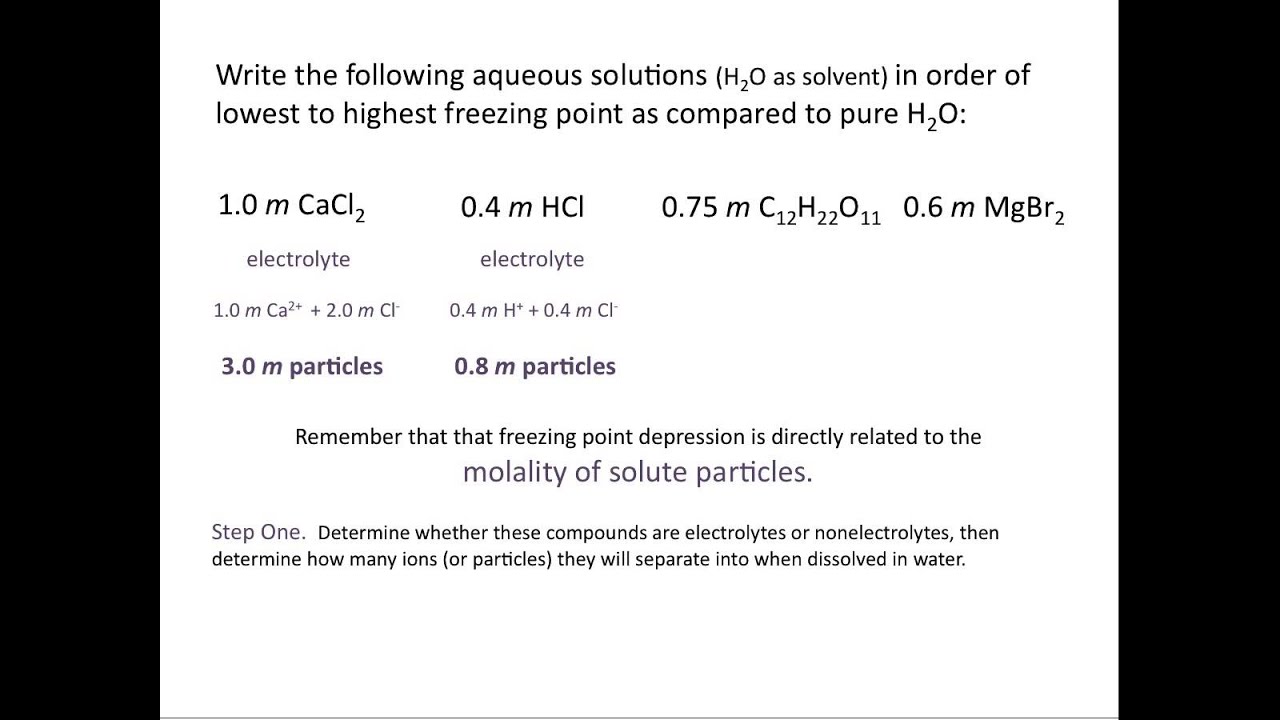
Which of the following would have the lowest freezing point. Freezing point of a pure solvent depends on. Aqueous sucrose 075 m d.
AWhich of the following solutions has lowest freezing point and why. Which of the following solutions have lowest freezing point. Among the given options R-22 has lowest freezing point.
A 01NaCL b 001 M NaCLc 1M NaCLd 0001M NaCL. Compared to the freezing point and boiling point of water at 1 atmosphere a solution of a salt and water at 1 atmosphere has a. Add your answer and earn points.
This is because it dissociates into 3 ions one Ba and 2 OH which is more than any of the others. Which of the following liquids will have the lowest freezing point. A 015M glucose b 030 M sucrose c 015 M NaCl d 030 M CH3COOH.
Which of the following liquids will have the lowest freezing point. AHO B N 2 C SO DH E COZ. Freezing point depression is a colligitive property of water that depends in part on the number of ions.
1 Al2SO43 2 C6H12O6 3 KI 4 K2SO4. 1m NaCl 15m CH3CH2OH 07m AlCl3 12m CsNO3. A pure H₂O B aqueous glucose 060 m C aqueous FeI₃ 024 m D aqueous KF 050 m E aqueous sucrose 060 m D aqueous KF 050 m Which of the following liquids will have the lowest freezing point.
Which of the following has the lowest freezing point. Which of the following solutions has the lowest freezing point and the highest boiling point. Lower freezing point and a higher boiling point.
Amelia4152 amelia4152 Remember the greater the concentration of particles the lower the freezing point will be. If seawater is an aqueous solution of sodium chloride calculate the molality of seawater. Which of the following refrigerants has lowest freezing point.
The k f for water is 186 K m. The reasons why are dictated by quantum mechanics. The zero point energy of a helium system is too great to allow freezing.
The material with the lowest freezing point is helium. Solved Which of the following has the lowest freezing point. A pure H2O B aqueous CoI2 0030 m C aqueous NaI 0030 m D.
A 02 m NaClO4 B 02 m MgClO42 C 02 m sucrose C12H22O11 D 02 m Na3PO4 E 02 m NaNO3 BWhat is the freezing point of a solution formed by dissolving 15 g AlCl3 in 150 g water. Mixing 176 kg of ethylene glycol commercial antifreeze C2H6O2 with 600 gal of water lowers the freezing point to 100 F. 022 100 points The freezing point of seawater is about - 185 C.
Which of the following solutions have lowest freezing point. Helium has the lowest freezing point. Ective molality is the highest in the group and therefore will have the lowest fp the highest bp and the maximum osmotic pressure.
Solution By Examveda Team R-22vhas the lowest freezing point. Aqueous lif 065 m b. Which of the following 01 M aqueous solutions will have the lowest freezing pointA.
Higher freezing point and a lower boiling point. Under typical pressures it does not freeze at all even at temperatures approaching absolute zero. Which of the following aqueous solutions has the lowest freezing point.
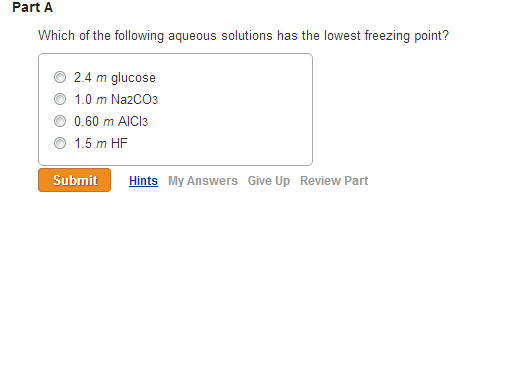
Simply find the solution with the highest value of mxi a. Which of the following has the lowest freezing point. 0102 m sucrose 0050 m NaCI b.
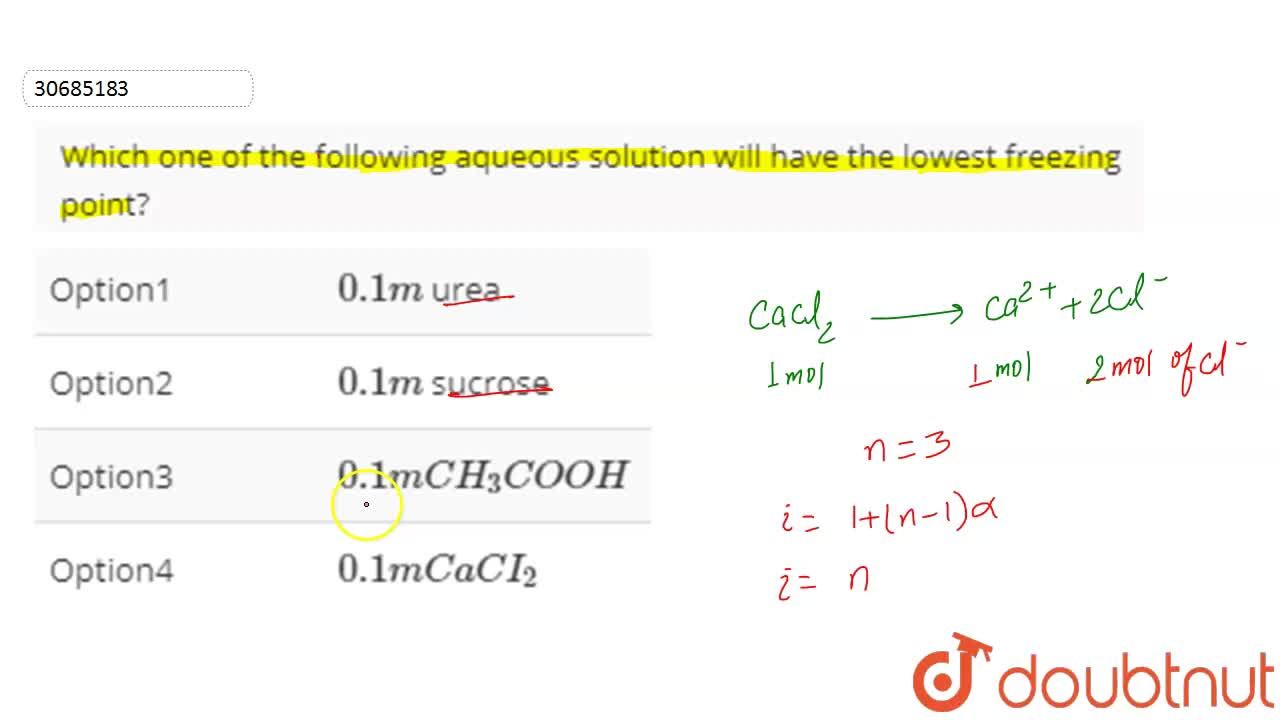
01mCaI2 will have the lowest freezing point followed by 01mNaCl and the highest of the three solutions will be 01mC6H12O6 but all three of them will have a lower freezing point than pure water. Which of the following solutions will have the lowest freezing point.

Freezing Point Depression Chemistry Tutorial Youtube

Which One Of The Following Solutions Has The Lowest Freezing Point

Which Of The Following Aqueous Solutions Will Have The Lowest Freezing Point
Solved Which Of The Following Has The Lowest Freezing Point Chegg Com

Solved 13 Which Of The Following Solutions Will Have The Chegg Com

Quick Answer What Element Has The Highest Freezing Point Seniorcare2share

Calculating Freezing Point Depression Youtube

Fahrenheit Wikipedia The Free Encyclopedia Sensible Heat Chemistry Lessons Thermometer

Temperature Conversions Temperatures Conversation Problem Solving

Which Of The Following 0 1 M Aqueous Solutions Will Have The Lowest Freezing Point Youtube

Solved Which Of The Following Aqueous Solutions Has The Chegg Com

Which One Of The Following Aqueous Solution Will Have The Lowest Freezing Point

Calculating Freezing Point Depression Youtube

Solved Which Of The Following Has The Lowest Freezing Point Chegg Com

Colligative Properties Ppt Video Online Download

Which Has Lowest Freezing Point Seniorcare2share
Freezing Point Depression Chemistry Tutorial Youtube

Which Of The Following 0 1m Aqueos Solution Will Have The Lowest Freezing Point K2so4 Nacl Urea And Glucose Chemistry Solutions 13781011 Meritnation Com
Solved Which Of The Following Aqueous Solutions Has The Chegg Com
Comments
Post a Comment